Research
From Molecular Mechanism to Cellular Morphology
Our goal is to elucidate the biochemical and biophysical principles that underlie the self-organization and scaling of subcellular organelles. Each cellular organelle carries out a distinct function, which is not only related to its molecular composition, but in many cases also to its size. The mitotic spindle for example must be large enough to span sufficient distance to physically separate chromosomes into two opposite halves of the cell. To understand the higher-order structure and dynamics of the metaphase spindle we need to study and link processes on several hierarchical length scales.
Nanometre Scale
On the smallest length scale, we wish to understand the molecular mechanisms by which proteins function. In particular, we are interested in proteins that nucleate, grow, cross-link, and move (along) microtubules. Our approach towards understanding a protein’s function includes protein engineering, single-molecule studies and structure-function analyses using purified components.
The video on the left shows dynamic microtubules (in green) growing from stable microtubule seeds (in red) as seen by Total Internal Reflection Fluorescence (TIRF) Microscopy.
Micrometre Scale
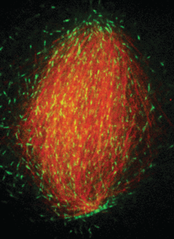
To further our understanding of the mesoscale organization of the spindle, we study how microtubule dynamics and forces translate into a given spindle size and shape. As a model system, we use Xenopus (linked to frogs) egg extracts, which allows us to reconstitute a functional metaphase spindle. A key advantage of the Xenopus egg extract is its biochemical accessibility that permits quantitative kinetic studies. Moreover, the extract is a cell-free system void of cortical restrictions, which allows us to study intrinsic mechanisms of length control.
The figure on the right shows an in vitro reconstituted spindle with microtubules shown in red and the protein EB1, which tracks growing microtubule ends, is shown in green.
Millimetre Scale
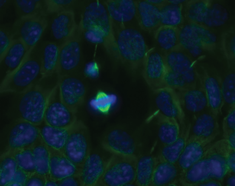
On the larger length scales, our long-term goal is to understand how subcellular structures, such as the mitotic spindle, scale with cell size. In particular, we wish to dissect the scaling principles throughout development and differentiation. As a model system, we use mouse embryonic stem cells (mESCs) and take advantage of their potential to differentiate into specific cell lineages using the emerging techniques of BAC transgenesis.
The figure on the left shows mouse embryonic stem cells. The cell in the centre is undergoing mitosis, spindle microtubules are shown in green and the chromosomes in blue.
Systems Biology
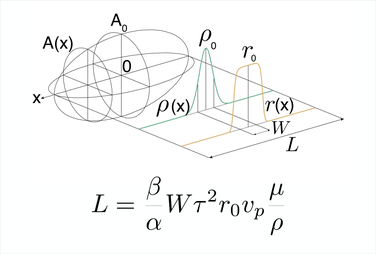
In order to achieve a systems understanding of organelle organization, we need to link observations from all length scales. In particular, we aim to link the function of single proteins to microtubule dynamics, to the overall spindle size and shape and ultimately to cell size. Theoretical and conceptual approaches are powerful tools to describe complex biological systems and dynamic processes and link them to measured quantities.
The schematic on the right represents a quantitative description of spindle length by microtubule mass balance.